❄PERPACHILL: FIRST IN COLD THERAPY❄
Huge Cold Capacity that Lasts Longer than Ordinary Gel Packs
Pain and inflammation relief solutions
The best way to treat the pain and swelling experienced following an injury is to apply cold to the injured area. Often patients will use painkillers to deal with discomfort relating to their injury, but using this method is dangerous. Painkillers do nothing to treat the pain or inflammation associated with an injury, merely covering up the damage or even making the injury worse. Instead of painkillers, use cold to relieve this pain safely and effectively.
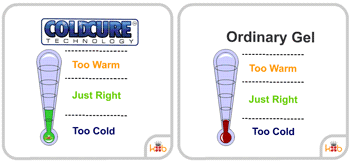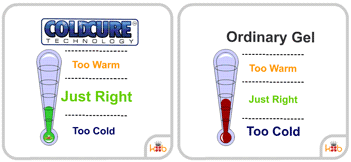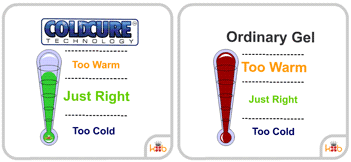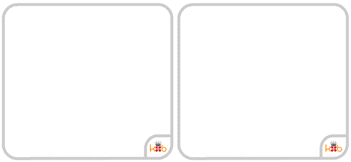
Many wraps will not stay cold for very long and will quickly lose their ability to reduce pain and swelling. ColdCure® wraps, however, use Perpachill® technology to keep the gel packs cold. Perpachill® gives the RigiGel® gel in every ColdCure® wrap the power to hold the cold longer than any other wrap. This means you can continue treating your injury for much more time than most gels.
The problem with many ice gels is that they do not properly distribute cold evenly over the whole injury, and do not stay cold for long. The way to fix this problem is to use a ColdCure® cold compression wrap. ColdCure® wraps use thicker, bigger wraps that hold their shape to keep the cold on the injury.
Increased Cold Capacity
Most ice packs or gels only last for a short while before they lose their temperature and cease being being useful for treatment. ColdCure® wraps employ Perpachill® technology to stay cold for much longer than ordinary wraps. It's because of this that Perpachill® gels have up to 10x the cold power compared to conventional gels.
ColdCure® wraps with Perpachill® are proven to have pound-for-pound triple the cold power of leading pharmacy wraps.
➙Click here for more information on ColdCure treatments!Use Cold, not Ice
It's common for many people to put ice on their injuries to relieve pain and keep swelling down. However, they are only putting themselves at risk of further damage to their tissue. Ice from the freezer is too cold and can often burn the skin over the injury. To solve this, ColdCure® RigiGel® is designed to be stored in the fridge, where it is not too cold to burn but is more than enough to treat pain and inflammation. There's no need to put yourself at further risk.
Quality Wraps
Each ColdCure® wrap is made of soft, comfortable Neoprene. They are designed with quality in mind for patients to enjoy wearing. The wraps are built to be injury-specific; there is a variety of wraps in shapes that fit the forms of many common injured body parts such as the knee or shoulder. Their unique shape allows for the best and most comfortable coverage for the entire injured area.
Paired with Revolutionary ColdCure® Wraps
Perpachill® technology is one of the key components in the design of ColdCure® wraps. The wraps combine the benefits of cold therapy with compression and comfort, improving on all the models to create the ultimate pain and swelling solution. It's reccomended that patients start using a ColdCure® wrap following an injury to alleviate the discomfort and swelling. With the cold and compression aspects working together along with the powerful Perpachill® and RigiGel® technologies, ColdCure® provides the best possible solution to post-injury pain and swelling.
➙Click here for more information on ColdCure treatments!Forum Content from the Health Care Company King Brand®
To treat a Baker's Cyst, should I get the Knee ColdCure or the Leg ColdCure?
A customer asked, "To treat a Baker's Cyst, should I get the Knee ColdCure or the Leg ColdCure?"
Re: To treat a Baker's Cyst, should I get the Knee ColdCure or the Leg ColdCure?
To treat a Baker's Cyst, we recommend the Leg ColdCure wrap. The reason is because the Leg ColdCure wrap has an effective treatment area of 10" x 7", which is capable of wrapping around the back of the knee, and applying cold compression to the back of the knee, which is typically where a Baker's cyst is found.
The Knee ColdCure wrap can still be used to treat a Baker's cyst, it would just need to be rotated around so that the cold therapy applies to the back of the knee - the fit will not be ideal on the back of the knee, as the Knee ColdCure is designed specifically to treat the front of the knee.
The Leg ColdCure is most ideal for a Baker's cyst because it provides a better fit. The Leg ColdCure is a more versatile wrap and can be used to treat any area of the leg or hip.
You can find the products we recommend specifically to treat a Baker's cyst by clicking the link below.
http://shop.kingbrand.com/product_info.php?products_id=331&REF=2160PV0.1539
→ Click here for the the full King Brand® Forum
